Nasty nanoinjectors pose a new target for antibiotic research
LAWRENCE — If you’ve ever suffered the misery of food poisoning from a bacterium like Shigella or Salmonella, then your cells have been on the receiving end of “nanoinjectors” — microscopic spikes made from proteins through which pathogens secrete effector proteins into human host cells, causing infection.
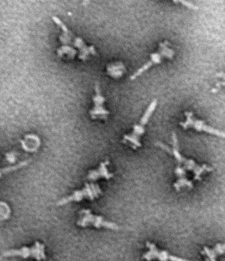 Many bacteria use nanoinjectors to infect millions of people around the world every year.
Many bacteria use nanoinjectors to infect millions of people around the world every year.
Today, Roberto De Guzman, associate professor of molecular biosciences at the University of Kansas, is leading a research group that is evaluating the potential of nanoinjectors as a target for a new class of antibiotics. Their work is funded by a five-year, $1.8 million grant from the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health.
“This grant will support our studies on elucidating how bacterial nanoinjectors are assembled,” said De Guzman. “Nanoinjectors are protein machinery used by bacterial pathogens to inject virulence proteins into human cells to cause infectious diseases. They are nanoscale is size — they look like needles and bacteria use them to inject virulence proteins into host cells — so I called them nanoinjectors. In microbiology, they are known as part of the type III secretion system, a protein delivery machinery.”
The KU researcher said nanoinjectors are unique to pathogenic bacteria and are absolutely required for infectivity. Most people have heard of the diseases caused by bacterial pathogens that employ nanoinjectors — several of which have changed the course of the human experience for the worse.
“Examples are Yersinia pestis, which caused the Black Death in Europe and altered world history,” said De Guzman. “Also, Pseudomonas aeruginosa, the number one cause of mortality among cystic fibrosis patients and a major source of secondary hospital infections, and Chlamydia, a major source of bacterial sexually transmitted disease.”
Because an increasing number of pathogens have evolved strains that are unaffected by antibiotics now on the market, De Guzman said that new approaches in drug development are necessary — and nanoinjectors could present a worthwhile target.
“The problem is that all of these pathogens have developed resistance to current antibiotics,” he said. “Further, antibiotics are not as profitable as other drugs, so pharmaceutical companies have disfavored developing them. Hence, there is a dearth of new antibiotics in the pipeline. We’re in for a perfect storm when the age of antibiotics is no longer assured.”
A major factor in NIH awarding this grant to KU is a $1.9 million nuclear magnetic resonance or NMR spectrometer — essentially a huge magnet — that the university bought in 2004 through a bond approved by the Kansas Legislature.
“We have the critical instrument needed for this research,” said De Guzman. “This is my second major NIH grant, plus the other grants received by KU that rely on the NMR magnet. I think Kansas got its money’s worth many fold on that investment.”
Using the NMR spectrometer, De Guzman and his team hope to better understand the biological processes that create nanoinjectors.
“Nanoinjectors are assembled from about 20 different types of proteins, and parts thereof — like the needle itself and proteins associated with the needle — are surface-exposed,” said De Guzman. “The nanoinjector is assembled in precise manner where proteins come together like tight-fitting Lego blocks. A tiny defect could render the whole thing useless for pathogens making them non-infective.”
The eventual goal is to find or develop compounds that promote such defects in nanoinjectors, rendering the pathogens harmless to humans.
“My interest is to understand in atomic detail how the needle is assembled and extend that knowledge into developing drugs that will disrupt the assembly of the nanoinjectors and thereby prevent pathogens from infecting their hosts,” said De Guzman.
Note: Image, courtesy of Dr. Matthew Lefebre and Professor Jorge Galan (Yale University), shows parts of nanoinjectors from Salmonella as seen under an electron microscope.